How it works, and how what we eat and how we eat it influences our health
Normal eating behavior is coordinated by a tightly regulated balance between intestinal, extraintestinal and central homeostatic and hedonic mechanisms, resulting in stable body weight.
Dr. Alfonso Galán González – Neolife Medical Team
The influence of chronic stress on weight gain
We are experiencing a true obesity epidemic in the western world and its consequences at the metabolic level are a major problem for health systems.
Its causes are multifactorial, but today we are going to focus on the dysregulation of the brain-gut-microbiota (BGM) axis, which plays a leading role.
We will see how the chronic stress we experience in many cases may influence the levels of the orexigenic hormone insulin, as well as glucose and cortisol responses, often leading to cravings and increased food intake and weight gain.
Normal eating behavior is coordinated by a tightly regulated balance between intestinal, extraintestinal and central homeostatic and hedonic mechanisms, resulting in stable body weight. What’s this about homeostatic vs hedonic mechanisms?
Homeostatic refers to consuming what we need to maintain our body weight and maintain the stability of our systems, i.e. “eating what we need”; while hedonic refers to those eating behaviors that seek to obtain pleasure. The ubiquitous availability and marketing of inexpensive, highly palatable, calorie-rich foods has played a crucial role in shifting this balance toward hedonic eating by both central (through disruptions in dopaminergic signaling) and gut (vagal afferent function, metabolic toxemia, systemic immune activation, changes in the microbiota) mechanisms.
The balance between homeostatic and hedonic eating behaviors is not only influenced by the amount and composition of the diet, but also by the timing and rhythm of food intake. Circadian rhythms affect both eating behavior and multiple intestinal functions, as well as the composition of our microbiota and its interactions with the gut.
The Nutritional Study of the Spanish Population (ENPE) shows that 53.6% of Spaniards are obese or overweight.
There are countless dietary recommendations to lose weight and reduce the cardiovascular risk (CVR) associated with overweight, such as metabolic syndrome, cardiovascular disease, high blood pressure, diabetes, high cholesterol, or systemic inflammation. The vast majority of diets seek to reduce the total or relative amount of macronutrients (fats, carbohydrates, proteins) without much consideration for the role of the microbiota in regulating the gut-brain axis.
More recently, changing the way we ingest food, without calorie restriction, the so-called time restricted eating (TRE), has been proposed as a more effective long-term strategy to combat obesity and its metabolic complications (Hatori et al.). TRE is a combination of a ketogenic state during which there is a period of 16-18 hours without food intake (including sleep) and the benefits of a healthy, primarily plant-based diet during the 6-8 hours of eating period, showing promising results as a sustainable strategy for maintaining optimal metabolic function. However, although numerous preclinical studies have demonstrated the effects of TRE on gut microbiota and body weight, to date there is insufficient clinical evidence to evaluate its long-term clinical effectiveness (Lowe et al.).
The brain-gut-microbiota axis and obesity
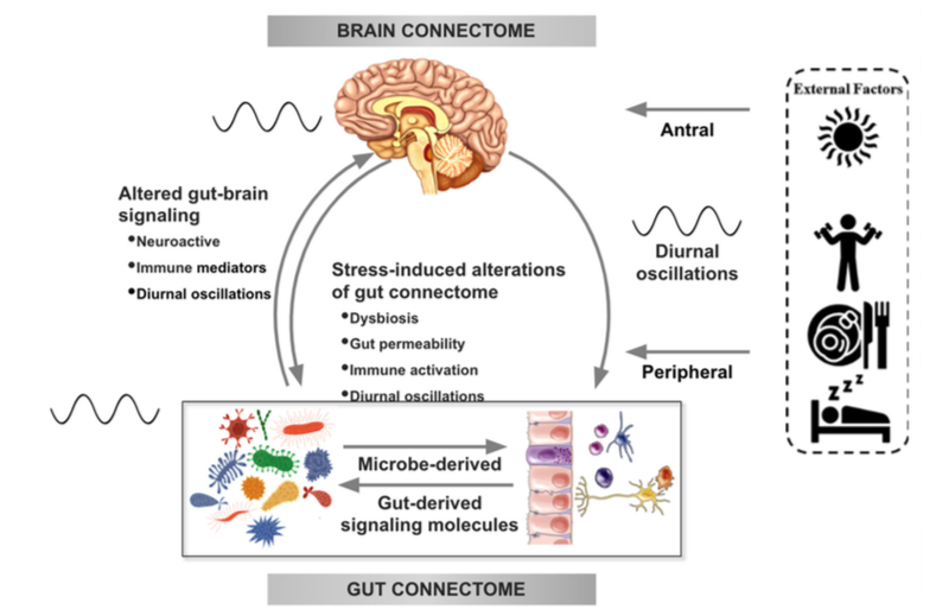
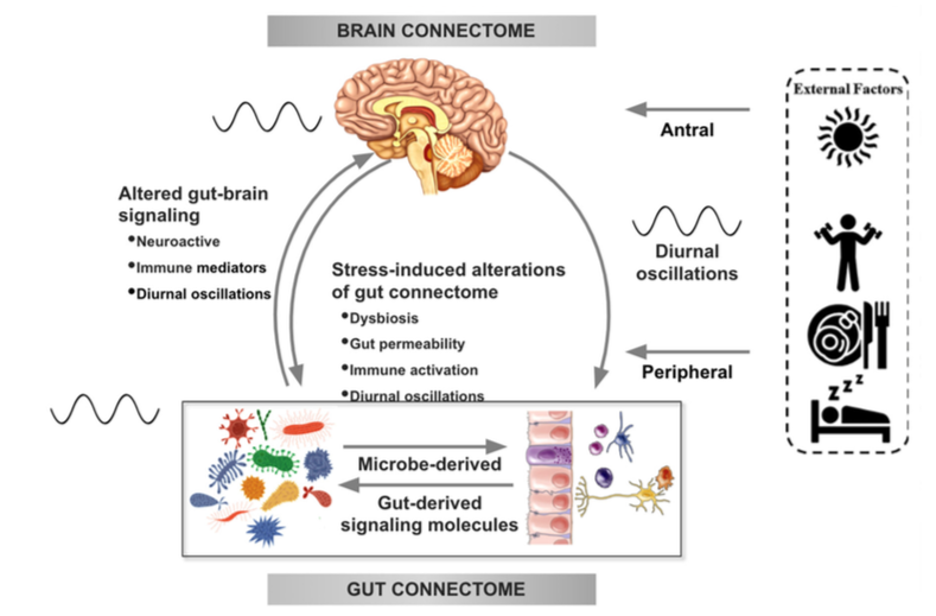
There is increasing evidence from preclinical studies supporting alterations in bidirectional signaling within the brain-gut-microbiota system in the pathophysiology of obesity. This signaling may be mediated by metabolic, endocrine, neural, and immune mechanisms (Gupta et al.). For example, specialized cells such as enteroendocrine and enterochromaffin cells in the intestine, which can detect many of the metabolites (such as short-chain fatty acids) produced by intestinal bacteria from ingested dietary fiber, send signals to the brain via the bloodstream or through vagal afferent pathways.
Signaling from the gut to the brain can also produce interactions between intestinal bacteria and intestinal immune cells. These interactions may produce local effects in the intestine, effects on vagal nerve terminals or cause systemic immune activation (metabolic toxemia), which may ultimately affect other organs and cells in the body, such as brain cells, leading to neuroinflammation.
In the other direction, brain signaling pathways that can modulate the gut and its microbiota include the autonomic nervous system (ANS), which is responsible for regulating gut processes such as immune activation, intestinal permeability, microbial abundance, and microbial gene expression in response to internal or external perturbations.
These various bidirectional signaling mechanisms between the brain and the gut allow for extensive communication within the BGM system, ensuring high adaptability to different dietary patterns and to different emotional and environmental states.
Under normal conditions, the BGM system plays a prominent role in regulating ingestive behaviors in a way that maintains stable body weight by balancing the body’s metabolic needs with the hedonic impulses of the central reward system.
There is extensive literature on the connection between homeostatic food intake and body weight maintenance through interactions between hypothalamic nuclei and gut orexigenic (that make us eat) and anorexigenic (that make us feel satiated) hormones, as well as chemical signals generated by the gut microbiota or derived from adipose tissue, specifically leptin.
The complex balance between orexigenic (ghrelin, insulin) and anorexigenic (cholecystokinin, neuropeptide-Y, glucagon-like peptide-1, short-chain fatty acids, leptin) signals, stress mediators (ACTH, norepinephrine, cortisol), the central reward system, and prefrontal cortical inhibitory mechanisms determines the timing and amount of food we eat.
An alteration of this tightly regulated system with a reduction in anorexigenic and inhibitory signals resulting in a dominance of hedonic drives has been implicated in the development of predominantly hedonic eating behaviors, known as food addiction that manifests as recurrent cravings and eating beyond a person’s metabolic needs (Berthoud et al).
The effect of stress on the brain-gut-microbiota axis
It is common for people experiencing high levels of stress or chronic stress to have an increased appetite for “comfort foods” or cravings. These foods are usually rich in sugars, fats, and salt (Dallman et al). This change in eating behavior often leads to weight gain and metabolic syndrome.
For example, a study of 339 adults (mean BMI = 26.7 ± 5.4 kg / m2) showed that chronic stress may influence the levels of the orexigenic hormone insulin, as well as glucose and cortisol responses, often leading to cravings and increased food intake and weight gain (Chao et al.).
Although the consumption of “comfort foods,” which usually occurs after dinner may cause an immediate sense of perceived stress relief, many studies have shown that the ingestion of highly palatable foods actually leads to increased autonomic responses, an increase in cortisol and ghrelin levels, and may alter the hypothalamic-pituitary-adrenal (HPA) axis, which have been associated with increased cravings and unhealthy eating habits. Like sugary foods, salty and fatty comfort foods have a high addictive potential.
In several preclinical models, persistent and episodic psychosocial stress has been shown to lead to a reduction in the richness and diversity of the gut microbiota. Moreover, a reduction was observed in the relative abundance of several microbial species in particular such as Lactobacillus and Akkermansia, bacteria that have been shown to have advantageous effects in reducing the risk of obesity and other metabolic diseases (Depommier et al.).
We will end this first part of this series of articles in which we unravel this incredible interaction between the brain, the gut, and our gut bacteria. In upcoming articles, we will discuss what else we know about it and how we can intervene to combat the obesity pandemic that is currently plaguing us.
BIBLIOGRAFÍA
(1) Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860
(2) Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain-gut-microbiome interactions in obesity and food addiction. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 655–672.
(3) Berthoud, H.R.; Munzberg, H.; Morrison, C.D. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology 2017, 152, 1728–1738.
(4) Dallman, M.F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010, 21, 159–165.
(5) Chao, A.M.; Shaw, J.A.; Pearl, R.L.; Alamuddin, N.; Hopkins, C.M.; Bakizada, Z.M.; Berkowitz, R.I.; Wadden, T.A. Prevalence and psychosocial correlates of food addiction in persons with obesity seeking weight reduction. Compr. Psychiatry. 2017, 73, 97–104.
(6) Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103