The genome, metabolome, and microbiome of a supercentenarian: clues for healthy aging.
At Neolife, we are always attentive to cutting-edge advances in aging science, and today we want to share a study that is generating significant interest. It is “The multiomics blueprint of the individual with the most extreme lifespan,” recently published in Cell Reports Medicine by Santos-Pujol and colleagues. This work deeply analyzes the multiple biological layers (genome, epigenome, transcriptome, metabolome, proteome, and microbiome) of a supercentenarian in an effort to understand how it is possible to age so extensively while maintaining exceptional health.
Dr. Sánchez – Neolife Medical Team
Study context
The individual analyzed (referred to as “M116” in the paper) was, at the time, the oldest person with verified longevity (117 years and 168 days), making her an exceptional case for studying the mechanisms underlying extreme lifespan.
The research team analyzed blood, saliva, urine, and stool samples, applying multiple omics techniques to compare her biological profile with that of older adults who were not supercentenarians.
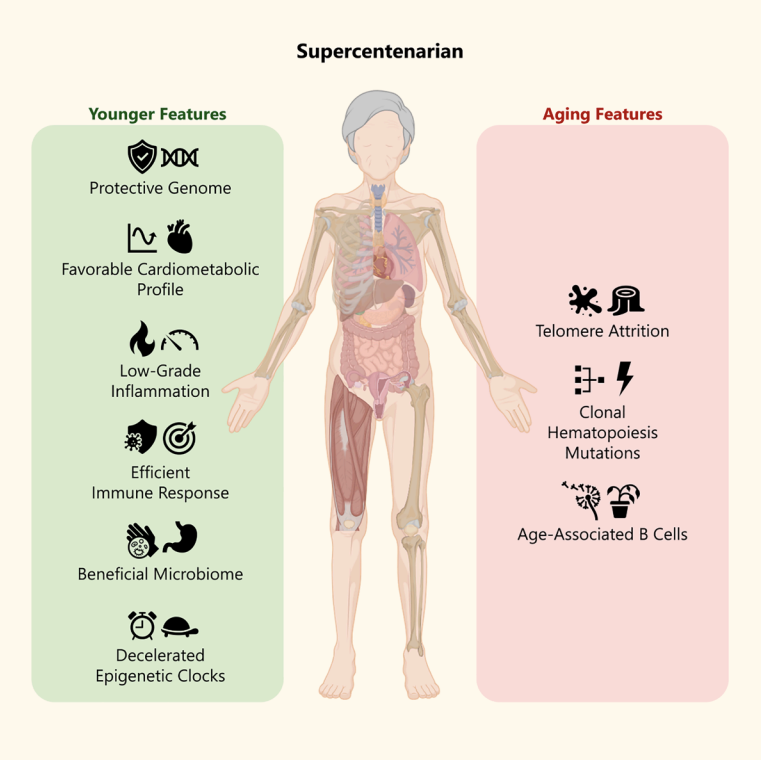
One key conclusion is that extreme longevity does not occur simply by “avoiding” aging, but rather through an intriguing duality: clear markers of advanced age (e.g., very short telomeres) coexisting with protective mechanisms that appear to preserve health.
In other words: she had not “defeated” aging, but had managed it in an exceptional way.
Key findings relevant to anti-aging practice at Neolife
Below we summarize the most important discoveries that, from Neolife’s perspective, are relevant to our clinical vision and guidance.
1. Genomics and rare variants
Researchers identified approximately 3.8 million single nucleotide variants (SNVs) in the woman’s genome, of which about 91,666 were considered “variants of interest” (VOI) with potential effects on more than 25,000 genes. Among these, seven were homozygous and absent from the European control population. Notable genes affected included: DSCAML1 (immunity and cognition)
MAP4K3 (longevity regulation in model organisms)
TSPYL4 and NT5DC1 (lung function)
The PCDHA1-9 cluster (brain and heart health)
Functional enrichment analysis showed overrepresented pathways such as:
T-cell differentiation in the thymus
Response to bacteria
Antigen-receptor–mediated signaling
Other immune-related mechanisms
This highlights the potential importance of a robust immune system in healthy longevity.
An interesting observation: although her telomeres were extremely short (~8 kb on average and ~40% below the 20th percentile), she remained in good health. This suggests that telomere length functions more as a “chronological clock” than a direct predictor of disease. At Neolife, this reinforces the idea that aging biomarkers must always be interpreted in a broad clinical context.
2. Metabolomics and lipid/anti-inflammatory profile
She displayed a remarkably favorable lipid profile: extremely low VLDL cholesterol and triglycerides, and very high HDL cholesterol (“good cholesterol”). Likewise, she had many medium/large HDL particles and many large LDL particles, with low levels of small HDL particles — all pointing to very efficient lipoprotein maturation.
She also showed low levels of saturated fatty acids, esterified cholesterol, linoleic acid, and acetone (typically associated with poorer health), and high levels of free cholesterol, which have been linked to better prognosis.
Her inflammatory profile showed low concentrations of glycoproteins A and B, suggesting a reduced degree of systemic “inflamm-aging.”
Altogether, these findings support the clinical value of interventions aimed at:
Optimizing lipid metabolism
Controlling triglycerides
Improving lipoprotein particle composition
Reducing low-grade chronic inflammation
All areas that Neolife actively addresses in personalized programs.
3. Extracellular vesicle (EV) proteomics
The proteomic analysis of M116’s extracellular vesicles (compared to postmenopausal women aged 49–65) revealed 231 proteins with significant differences. Enriched functional groups included:
Coagulation
Immune system
Lipid metabolism
Apoptosis
Cellular detoxification
Cell adhesion
mRNA regulation
Notable patterns included increased lipid and cholesterol transport, enhanced lipoprotein remodeling and clearance, and greater oxidative stress response activity.
A surprising detail: the most overexpressed protein in M116 was SAA1 (serum amyloid A-1), typically associated with Alzheimer risk, although she showed no signs of neurodegeneration. This underscores the need to interpret proteomic elevations with nuance and within the individual’s full biological context.
4. Gut microbiome
M116’s gut microbiome displayed much greater diversity compared to control women aged 61–91, indicating an extraordinarily rich microbial ecosystem.
At the phylum level, Actinobacteriota were markedly elevated, especially the family Bifidobacteriaceae and genus Bifidobacterium, which normally decline with age—but here remained abundant.
In contrast, Proteobacteria and Verrucomicrobiota were decreased relative to controls, patterns typically linked to better health in advanced age.
Dietary records showed that M116 consumed about three yogurts per day containing Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, which support Bifidobacterium growth. Although causality is not proven, these findings open practical avenues worth exploring.
5. Epigenetics and “biological age”
Globally, M116’s estimated biological age (based on epigenetic methylation clocks) was significantly lower than her chronological age. For example, her “age pace” was −17.34 years, suggesting a slowing of epigenetic aging.
This confirms the value of incorporating epigenetic clocks into aging assessments, while acknowledging their limitations: epigenetics is modifiable, and the goal is to realign biological age toward younger values.
Implications for healthy aging practice at Neolife
This study matters deeply to us at Neolife, and here is what it teaches when translated into clinical practice:
Extreme longevity is not the result of a single miraculous intervention, but of a synergistic set of genetic, metabolic, immune, microbiological, and epigenetic mechanisms. At Neolife, we emphasize exactly this comprehensive approach.
Efficient lipid profiles, low inflammation, a favorable microbiome, and slowed epigenetic aging emerge as “health markers” rather than indicators of chronological age. For this reason, we promote a personalized strategy: thorough diagnostics, tailored interventions (nutrition, microbiota modulation, lifestyle optimization, lipid and epigenetic management), and continuous monitoring.
This case demonstrates something powerful: extreme longevity does not have to be accompanied by extreme disease. Some individuals can live over a century while maintaining functional, remarkably balanced biology.
Although the study examines a single subject —and therefore cannot be fully generalized— it offers valuable hypotheses applicable to individualized clinical programs:
- Assessing lipid metabolism beyond traditional cholesterol measures (lipoprotein profiling, HDL/LDL particle analysis)
- Monitoring low-grade inflammation (glycoproteins, EV markers)
- Analyzing the gut microbiome with special attention to Bifidobacterium and reduction of pro-inflammatory taxa
- Integrating genomics into longevity assessments, recognizing that there are no “magic genes,” but rather many variants whose effects accumulate and are modulated by lifestyle
This research reinforces the idea that age is an important factor but not an unavoidable destiny: aging and disease can be decoupled, as shown by this case. In the authors’ own words, “extreme age” does not necessarily equate to “poor health.”
For us, it emphasizes the importance of analyzing multiple layers of biology —inflammation, genetics, epigenetics, microbiome— all of which add up. And above all, it reminds us that there is no single formula for successful aging, but there are measurable patterns we can understand and, to some extent, modulate.
We believe this study by Santos-Pujol et al. opens the door to future interventions in epigenetics, microbiome modulation, and personalized prevention. And although much remains to be explored, it reinforces our daily mission at Neolife: to translate cutting-edge science into actionable strategies that help patients preserve —and even elevate— their quality of life as they age.
Our commitment at Neolife is precisely to translate these cutting-edge advances into practical strategies for our patients, helping them optimize their aging instead of resigning themselves to the passage of time.
If you would like to explore any of these biomarkers in more depth or understand how to incorporate them into your follow-up protocol, we would be delighted to assist you.
Thank you for joining us on this journey toward smarter, healthier aging.
BIBLIOGRAPHY
(1) Santos-Pujol, A., Matalonga, J., Calleja, N., Moratal, T., López-Montes, A., et al. (2025).
The multiomics blueprint of the individual with the most extreme lifespan.
Cell Reports Medicine, 6, 102368.
https://doi.org/10.1016/j.xcrm.2025.102368