The combination of bioidentical estradiol and micronized progesterone has shown, according to scientific literature, no association with thrombotic events or an increased risk of breast cancer.
The warnings about hormone replacement therapy (HRT) date back to 2002, when the Women’s Health Initiative (WHI) study linked the combined therapy CEE + MPA (Conjugated Equine Estrogens + Medroxyprogesterone Acetate) to an increased risk of breast cancer and cardiovascular events.
Dr. Alfonso Galán – Neolife Medical Team
Women suffering from debilitating symptoms were kept away from an effective treatment due to unfounded fears, losing quality of life as well as bone and vascular protection.
In July 2025, the U.S. Food and Drug Administration (FDA) convened a panel of experts to reevaluate the evidence surrounding Hormone Replacement Therapy (HRT) in menopausal women. This marks a turning point in the institutional narrative that, for decades, has been dominated by fear, black box warnings, and a distorted perception of risk.
At Neolife, we welcome this step as an act of scientific and medical justice — but also as a reminder of the great harm that misinformation about HRT has caused. In this article, we analyze in depth the panel’s conclusions, the key studies discussed, and, above all, the fact that the formulations we use at Neolife (bioidentical estradiol and micronized progesterone) have shown no increase in cardiovascular or cancer risk.

What Was Said at the FDA Panel?
- Removal of the most severe warnings (black box warnings) for certain forms of HRT.
- Recognition of previously minimized benefits: cardiovascular protection, improved bone health, reduction of hot flashes, and enhanced mood—especially in women under 60 or within the first 10 years after menopause.
- Lack of differentiation between formulations: one major criticism was the failure to distinguish between treatments using conjugated equine estrogens (CEE) and medroxyprogesterone (MPA), versus bioidentical options.
The Legacy of the WHI and Its Consequences
The warnings about HRT trace back to 2002, when the Women’s Health Initiative linked CEE + MPA to an increased risk of breast cancer and cardiovascular events. However, in the years since, it has become clear that:
- The observed risk does not apply to all formulations or all women.
- The late age of treatment initiation (average age in WHI: 63 years) distorted the outcomes.
- The study did not use bioidentical estrogens or micronized progesterone.
As a result, millions of women gave up a beneficial therapy based on fears that we now consider unfounded for many patients.
Current Evidence: What Does Science Say About Bioidentical HRT?
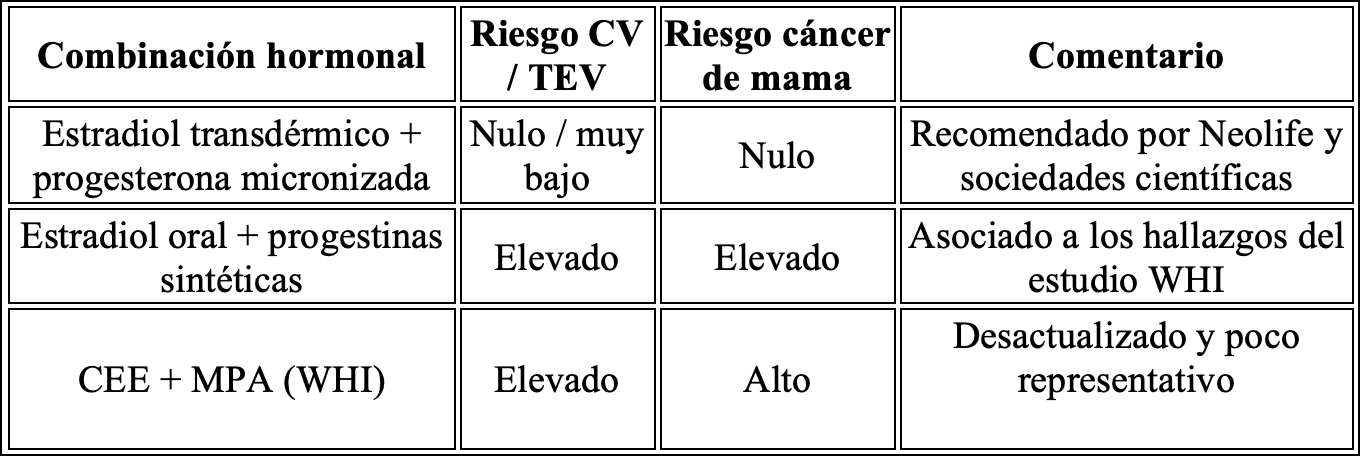
Studies such as E3N (France) have demonstrated that oral bioidentical estradiol combined with micronized progesterone does not increase breast cancer risk (RR: 0.9).
A meta-analysis (Scarabin, 2018) confirms that the safest combination is transdermal estradiol plus micronized progesterone, with no significant increase in thromboembolic risk.
Differences between formulations
The Neolife Approach: Evidence, Personalization, and Safety
At Neolife, we use HRT in a personalized and evidence-based manner, with:
- Bioidentical estradiol
- Micronized progesterone
- Regular clinical and laboratory monitoring
This combination has consistently shown no association with thrombotic events or increased breast cancer risk in the short- to medium-term scientific literature.
What Happens Next?
If the FDA is now willing to reconsider its most severe warnings based on current evidence, the next logical step would be to retract and acknowledge the harm caused over the past two decades.
Women with debilitating symptoms were denied an effective treatment due to misplaced fears, losing not only quality of life but also bone and vascular protection.
Medicine must have the courage to correct its course. Today, we know that well-prescribed, bioidentical, and personalized HRT is a powerful, effective, and safe tool for many women.
Conclusion
The FDA panel marks a shift in direction, but there is still a long road ahead — many physicians and patients remain to be educated. At Neolife, we have long applied an evidence-based approach, free from dogma and with safety as our top priority. It is time to rewrite the narrative of HRT — with science, prudence, and respect for women’s health.
BIBLIOGRAPHY
(1) Scarabin, P. Y. (2018). Progestogens and venous thromboembolism in menopausal women: an updated oral versus transdermal estrogen meta-analysis. Climacteric, 21(4), 341–345.
(2) Fournier, A. et al. (2008). Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. Journal of Clinical Oncology, 26(8), 1260–1268.
(E3N cohort, Francia)
(3) Manson, J. E. et al. (2013). Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA, 310(13), 1353–1368.
(4) The NAMS 2022 Hormone Therapy Position Statement Advisory Panel. (2022). The 2022 Hormone Therapy Position Statement of The North American Menopause Society. Menopause, 29(7), 767–794.
(5) FDA Expert Panel on Menopause and Hormone Replacement Therapy (2025). U.S. Food & Drug Administration Public Meeting Archive: July 17, 2025.

