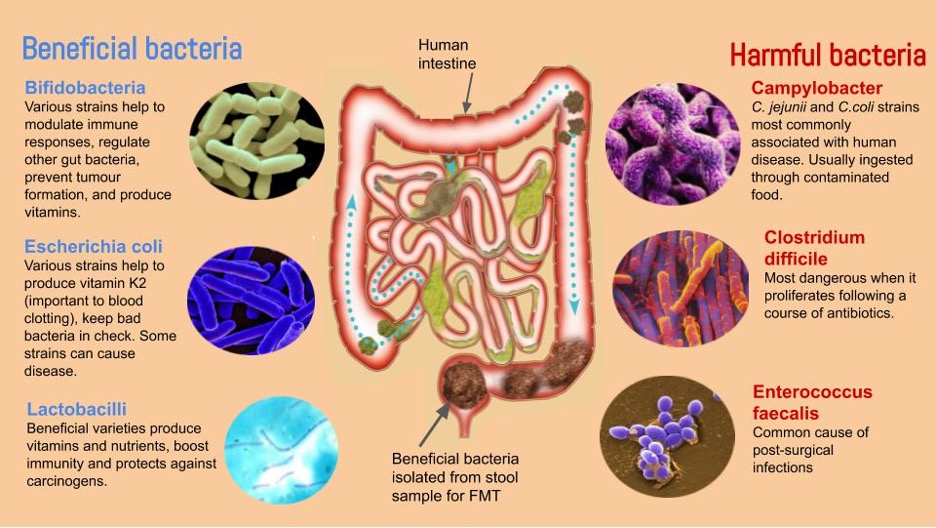
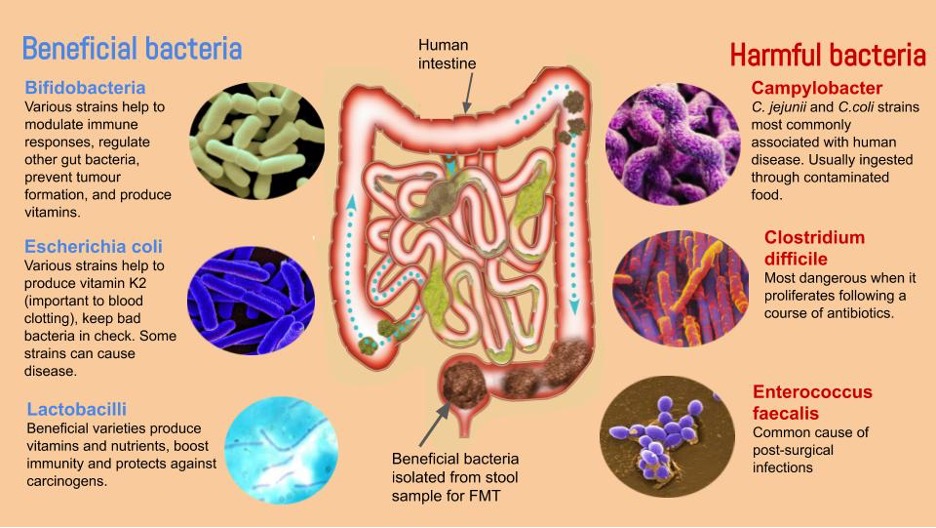
How it works, and how what we eat and how we eat it influences our health
Low levels of fiber in the diet cause intestinal microorganisms to consume the mucus layer lining the intestine, which compromises the intestinal barrier function and leads to what is known as “leaky gut”. This may result in inappropriate activation of the intestinal immune system and may lead to metabolic endotoxemia.
Dr. Alfonso Galán González – Neolife Medical Team
The effect of the immune activation of the gut microbiota on the Brain-Gut-Microbiota Axis in obesity
Studies on mice that were fed a high-fat diet and very low in dietary fiber have shown long-term alterations in gut microbiota diversity, while high-fiber diets resulted in positive alterations in eating behavior, such as decreased food intake and increased satiety. Limited fiber intake resulted in a substantial reduction in microbiota diversity and abundance, which was largely reversible in a single generation when animals received a fiber-rich diet. However, continuation of the low-fiber diet for several generations resulted in a permanent loss of microbial diversity, which was not recoverable even with increased fiber supplementation (Sonnenburg et al.).
Low dietary fiber levels cause intestinal microorganisms, such as certain strains of Akkermansia muciniphila, to consume the mucin glycans in the mucus layer lining the intestine, compromising intestinal barrier function and leading to a condition known as “leaky gut”. Reduced intestinal barrier function may result in inappropriate activation of the intestinal immune system. When inflammatory mediators spread beyond the gut, systemic immune activation can occur, affecting multiple organs, including the brain, a state that has been termed metabolic endotoxemia (Cani et al.).
This state of metabolic endotoxemia has been shown to decrease central satiety mechanisms by influencing the secretion by enteroendocrine cells of satiating hormones such as PYY, cholecystokinin, and 5-HT, as well as reducing the expression of anorexigenic receptors in vagal afferents and in the hypothalamus.
This state of metabolic endotoxemia has been shown to decrease central satiety mechanisms by influencing the secretion by enteroendocrine cells of satiating hormones such as PYY, cholecystokinin, and 5-HT, as well as reducing the expression of anorexigenic receptors in vagal afferents and in the hypothalamus.
Circadian variations of BGM interactions
The time of day plays an important role in metabolism and energy processes, as well as in most physiological functions. Much of mammalian behavior and physiological functions are organized around circadian rhythms, including feeding behavior and digestive system activity, such as motility and secretory patterns. The suprachiasmatic nucleus (SCN), a small region of the brain located in the hypothalamus, is considered the “master clock”, responsible for pacing and synchronizing all circadian rhythms in mammals. Disruption of the circadian rhythm may affect gastrointestinal function, the metabolism, and eating behaviors, and a considerable body of preclinical and clinical studies has demonstrated its association with an increased risk of chronic diseases, particularly metabolic syndrome, obesity, cardiovascular disease, and cancer.
In developed countries, the ubiquitous availability of food at all hours of the day, long-distance air travel across different time zones, and shift work have led to significant disruptions in circadian rhythms in terms of food intake. Specifically, the expansion of the window of time during which food is consumed, including late-night snacking, causes disturbances in the circadian rhythms of the Brain-Gut-Microbiota (BGM) interactions, which contributes to compromised metabolic function (Racz et al.).
The gut microbiota itself follows diurnal oscillations in composition and function and regulation is marked by the feeding rhythms of the host (Thaiss et al.). Therefore, the disruption of this balance between the timing of intake and circadian rhythms may lead to dysbiosis that may contribute to obesity and other metabolic dysfunctions.
Therapeutic implications
From what has been explained, it seems more than reasonable to use this evidence to develop strategies to combat obesity and provide better results than the usual ones based on modifying the macronutrient composition of the diet, which do not take into account the role of microbiota in developing resistance to weight loss.
Microbiota-targeted therapies
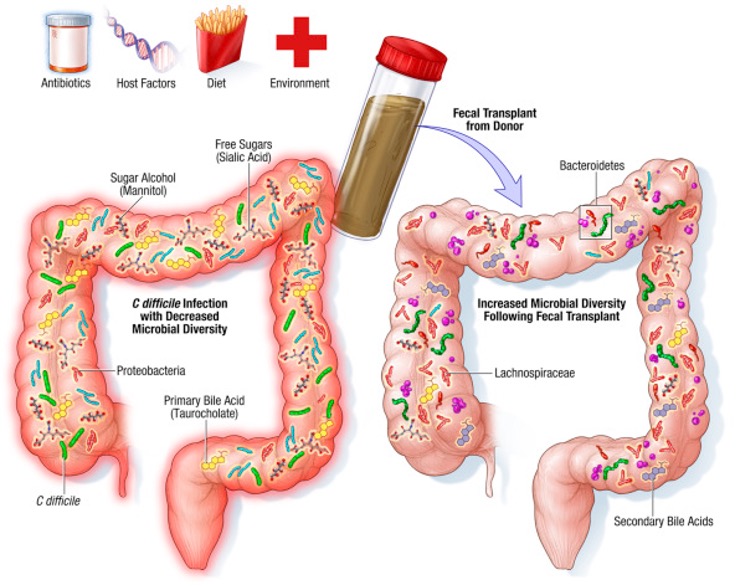
Microbiota-targeted therapies, such as treatment with novel probiotics or fecal microbiota transplantation (FMT), represent a new therapeutic option for obesity and metabolic syndrome. Small clinical studies have show good results.
Thus, in a small study of 18 individuals, where 9 received autologous FMT (from their own feces), and the other 9 received allogeneic FMT from a lean donor, and a second very similar study of 38 individuals, it was demonstrated that fecal microbiota transplantation from a lean donor increased butyrate-producing bacteria and improved insulin sensitivity in those recipients with metabolic syndrome (Vrieze et al.). However, the improved insulin sensitivity and associated changes in fecal microbiota were not maintained at 18 weeks of follow-up, due to the resilience of the recipient’s original microbiota.
Microbial products such as short-chain fatty acids (SCFA) are known to regulate feeding behavior in animal models through central mechanisms. For example, the consumption of a specific type of fiber that selectively increases propionate production by gut bacteria was correlated with a lower signaling of temptation and reward circuits in response to images of tasty food in 20 healthy non-obese men (Byrne et al.).
In order for these methods involving alterations in the gut microbiota to be sustained in the long term, dietary and behavioral changes need to be maintained over time so that the gut microbiota is fed with the nutrients necessary to continue the positive changes.
Temporary eating restrictions
Under the umbrella of the term intermittent fasting (IF), we have several forms of calorie restriction, such as alternate-day fasting and time-restricted eating.
Many recent studies have shown that metabolic pathways have diurnal rhythms. We believe that, under normal conditions, the cyclic expression of metabolic regulators coordinates a variety of cellular processes for more efficient metabolism.
The literature also shows us that IF may increase longevity, improve metabolic health, and help control hormonal changes, inflammatory reactions, lipid metabolism, and insulin sensitivity.
Time-restricted eating (TRE) is another proposed therapeutic approach to obesity and metabolic dysregulation that has gained significant attention in recent years (De Cabo et al.). TRE restricts food intake to a 6 to 8-hour window for a period of 24 hours with no calorie intake outside this window, as opposed to the 15 to 17- hour window of food intake that is common in Western countries.
The implementation of TRE where energy intake is limited to an 8-hour window prevents the harmful effects of metabolic diseases caused by high-fat and high-sugar diets without limiting daily calorie intake.
Based largely on studies in laboratory mice, it is hypothesized that time-restricted eating influences metabolic regulation through its effects on circadian rhythms, gut microbiota, and lifestyle.
In animals, TRE reduces fat accumulation and whole-body inflammation while improving glucose tolerance, reducing insulin resistance, and improving cholesterol control.
Allowing the digestive tract to have periods of time without food forces the body to burn fat during these periods instead of using energy from a continuous supply of glucose. In the absence of food intake, a metabolic shift occurs that forces the liver to produce ketone bodies from body fat when glucose is inaccessible. In addition to acting as fuel, ketone bodies are signaling molecules that have significant effects on multiple cellular and organ functions, including the brain.
It is believed that these systemic and cellular responses that are activated during fasting remain activated and strengthen mental and physical functioning, as well as resistance to disease even after resuming food intake.
Other potential factors that may contribute to the benefits of TRE include a subconscious reduction in snacking and overall calorie intake and changes in the gut microbial environment due to increased motility and secretion during the fasting period.
Despite impressive results in rodents, to date, the results of well-designed clinical trials to determine the effectiveness of this temporarily restricted food intake with or without calorie restriction in obese subjects with metabolic disturbances are, unfortunately, limited and inconsistent.
A study of overweight but otherwise healthy individuals who adhered to TRE significantly reduced their daily calorie intake primarily by eliminating alcohol and late-night snacking, resulting in sustained weight loss for up to one year (Gill et al.).
The randomized clinical trial TREAT, published in JAMA Internal Medicine in 2020, tested the effects of TRE on weight loss and other metabolic parameters in obese women and men (participants had a mean weight of 99.2 kg and a mean BMI of 32.7). The TRE group followed an eating schedule of 16: 8 hours and was instructed to eat whatever they wanted from 12 p.m. to 8 p.m. and to abstain completely from calorie intake from 8 p.m. to 12 p.m. the following day. The group with conventional meal timing (CMT) was instructed to eat three structured meals per day.
The investigators hypothesized that since TRE does not require a decrease in calorie intake in the 24-hour period, it must affect energy expenditure to achieve a negative calorie balance.
The primary outcome of TRE in the trial was significant weight loss in the TRE group and little to no change in weight in the CMT group. They also found a significant difference in appendicular lean mass index (in limbs) between the groups, but no change in other secondary outcomes such as fasting insulin, fasting glucose, or estimated calorie intake.
The study concluded that, without other interventions, TRE is not necessarily more effective for weight loss than daytime eating and may cause loss of lean muscle mass (Lowe et al.); however, previous studies with time-restricted eating in overweight or obese humans showed a reduction in total calorie intake leading to a decrease in body weight and fat mass.
According to the results of the TREAT study, adherence to a regular physical exercise program and a healthy diet can prevent these unexpected side effects such as loss of lean mass.
More research in humans is needed to draw more definitive conclusions about TRE, but it seems that the way forward is to combine it with a healthy diet during the period of eating with good protein intake and associated with a regular physical exercise program to avoid loss of muscle mass.
BIBLIOGRAPHY
(1) Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772.
(2) Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K.; Wade, P.A. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018, 19, 7.
(3) Racz, B.; Duskova, M.; Starka, L.; Hainer, V.; Kunesova, M. Links between the circadian rhythm, obesity and the microbiome. Physiol. Res. 2018, 67, S409–S420.
(4) Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529.
(5) Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916e917
(6) Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14
(7) de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551.
(8) Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798.
(9) Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499
(10) Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215