It is very important to know how efficient our cells are when producing ATP (adenosine triphosphate), a process which depends on how efficient each mitochondria is, how many normo-functional mitochondria there are, and how effective the cell is at eliminating the useless mitochondria. We could define mitochondrial dysfunction as a state in which ATP production by our mitochondria decreases. Mitochondrial dysfunction directly or indirectly affects all chronic diseases associated with aging.
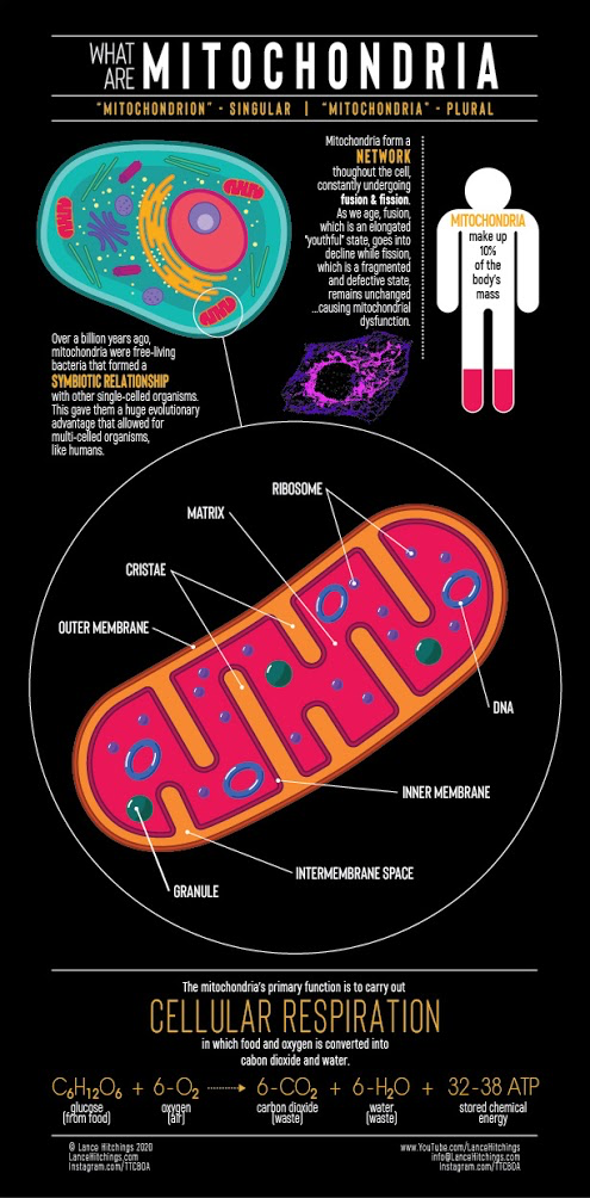
Mitochondria are very special organelles within our cells, so much so that they even have their own genetic material (mitochondrial DNA), and they are, among other important functions, responsible for producing ATP through a process called cellular respiration in which the nutrients we ingest and absorb are combined with the O2 we breathe to convert them into CO2 and H2O, producing ATP along the way.
Dr. Alfonso Galán González – Neolife Medical Team
Mitochondrial dysfunction is at the bottom of metabolic syndrome, cancer, cardiovascular disease, autoimmune diseases, sarcopenia, fatigue, etc. It also has a close relationship with inflammation and immunity.
We have talked in detail in these articles about the 9 “Hallmarks of Aging” that can be seen in the figure below.
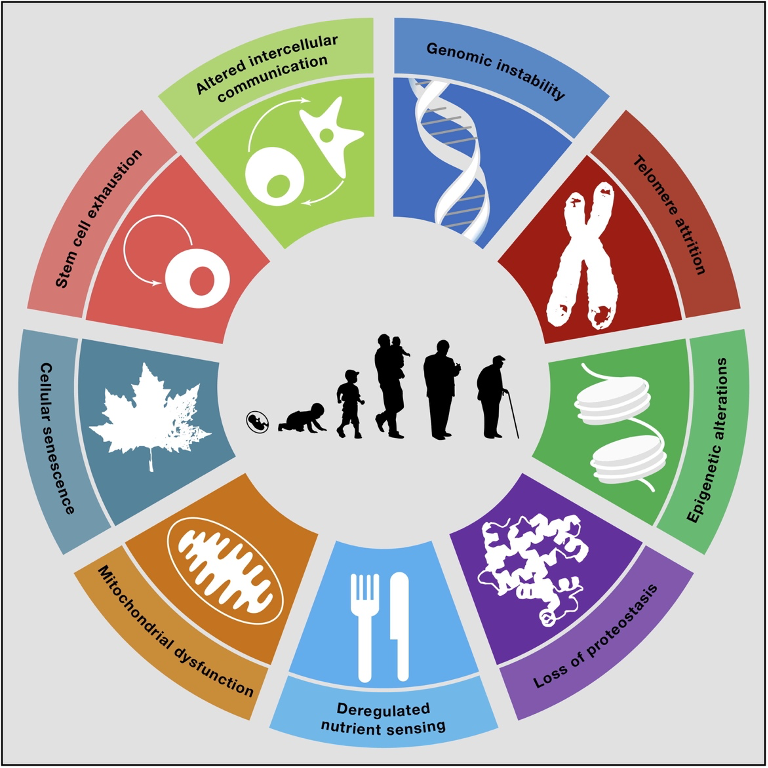
These are the 9 mechanisms that science recognizes as causing aging at the molecular and cellular level, as well asdiseases associated with aging such as osteoarthritis, osteoporosis, cardiovascular disease, cancer, diabetes, Alzheimer’s, Parkinson’s, etc.
As I am writing these lines, science is advancing to seek ways to “combat” each of these mechanisms. For many of them we already have effective ways to intervene that have been proven in experiments of different scopes (at the laboratory level, experiments in animals, and experiments in humans). The challenge is to find solutions that are safe and viable in humans.
But we also wonder: Are any of these mechanisms more important than the others? Would it be interesting to focus our efforts more on one rather than another? If I have to treat just one, which one should I choose?
Here, we are going to present the candidacy of mitochondrial dysfunction.
What is mitochondrial dysfunction? What relationship do they have with ageing?
Mitochondria are very special organelles within our cells, so special that they even have their own genetic material (mitochondrial DNA), and they are, among other important functions, responsible for producing ATP (adenosine triphosphate) through a process called cellular respiration in which the nutrients we ingest and absorb are combined with the O2 we breathe to convert them into CO2 and H2O, producing ATP along the way.
ATP is responsible for providing energy for 90% of the processes that take place in our body.
Therefore, it is very important to know how efficient our cells are when producing ATP, a process which depends on how efficient each mitochondria is, how many normo-functional mitochondria there are, and how effective the cell is at eliminating the useless mitochondria.
We could define mitochondrial dysfunction as a state in which ATP production by our mitochondria decreases.
When the production of ATP decreases, we have less energy to undertake our internal processes and the entire system slows down.
Mitochondrial dysfunction directly or indirectly affects all chronic diseases associated with aging.
It is estimated that the signs and symptoms of mitochondrial dysfunction come approximately 10 years before the problem actually sets in, some of those symptoms are:
- Fatigue
- Muscle weakness
- Loss of muscle coordination.
- Alterations in sight and hearing.
- Mental fog or cognitive problems, etc.
What are the causes of mitochondrial dysfunction?
Among the causes associated with lifestyle, nutrition plays a very important role. The worse we eat, the worse the state of our mitochondria will be and the worse they will be able to do their job. Physical activity also is a fundamental factor: as we get older, we tend to be more sedentary. The less demand we have to produce energy in the mitochondria, the less ATP it will produce and this will make our processes slower and we’ll be able to do less exercise, putting us into a very negative vicious circle.
Among the most precise causes of mitochondrial dysfunction, we can list:
- Changes in morphology.
- Alteration of balance between fusion and fission. Fusion is the process by which the smaller and somewhat damaged mitochondria join together with larger mitochondria to form a matrix where they share genetic material and which compensates for the loss of function that they may have. Fission, on the other hand, is the process activated by something called mitophagy, which is a special form of autophagy, mentioned in other entries on this blog, in which mitochondria are fragmented, something essential for removing damaged mitochondria. As we age, accumulated and a decrease in ATP production harm mitophagy and the balance between fusion and fission is broken, with a predominance of fusion over fission, and damaged mitochondria are not recycled as a result.
- Reactive oxygen species (ROS) increase. These occur as a consequence of these energy production processes when some of the electrons produced combine with oxygen, yielding these ROS that can damage mitochondrial DNA. Mitochondrial DNA has a much lower repair capacity than nuclear DNA and, therefore, has a much higher mutation rate.
- Levels of NAD+ (which is a fundamental electron acceptor for cellular respiration) decline as we age, affecting ATP production.
So, what importance does mitochondrial dysfunction have in the aging process and the development of illnesses?
Mitochondrial dysfunction is at the bottom of metabolic syndrome, cancer, cardiovascular disease, autoimmune diseases, sarcopenia, fatigue, etc.
It also has a close relationship with inflammation through something called mtDAMPs (mitochondria-derived damage-associated molecular patters), as it activates the release of proinflammatory cytokines. Likewise, it has a relationship with immunity, affecting innate immunity and T cells, with this being one of the reasons why older people are more susceptible to infections.
Why mitochondrial dysfunction?
Mitochondrial dysfunction is the target of our efforts because in one way or the other it is related with practically all the other 8 “Hallmarks of Aging” (González-Freire et al). For example, telomeric shortening is related to lower mitochondrial biogenesis (Sahin et al). The sirtuins, a group of enzymes that we have talked about on other occasions which are very related to altered nutrient sensitivity, influence autophagy/mitophagy and the recycling of damaged mitochondria, as well as the expression of many respiratory chain enzymes (Giralt et al). They also affect the production of ROS. D’Aquila et al. have additionally researched their relationship with epigenetic modifications.
What can we do?
Having seen all the negative aspects of mitochondrial dysfunction, one can get the idea that when it happens, all is lost. Fortunately, that is not the case. Mitochondrial dysfunction can be reversible if it is caught early enough. Interventions on the nutritional, supplementation, exercise, and hormonal levels have proven to improve mitochondrial functioning.
1. Nutrition
What we eat and how we eat it influences the functioning of our mitochondria a lot. We should eat non-processed, organic, and natural foods like meat, fish, dried fruits, seeds, eggs, and leafy green vegetables with vivid colors that are rich in antioxidants. Health fats like fish oil, avocado, coconut oil, olive, etc.
How we eat is also important. Intermittent fasting, as is known and as we have explained here , has great properties and here is yet another: it improves the fission/fusion balance, improves the burning of fatty acids, and activates the AMPk pathway (explained here), which is involved in obtaining energy in the form of ATP.
2. Supplementation
Various compounds have been shown to improve mitochondrial function, including:
- Group B vitamins.
- Vitamin C.
- Vitamin E
- Iron.
- Selenium.
- Zinc.
- Magnesium.
- Alpha lipoic acid.
- Coenzyme Q10.
- NAD in the form of its precursors, nicotinamide mononucleotide and nicotinamide riboside (NMN and NR).
- Ellagitannins present in red berries.
- Detoxifiers. Periodically undertaking a detox schedule to get rid of medicines, pollutants, etc. that have accumulated is beneficial for mitochondrial function.
3. Exercise
Exercising, staying active, and, more specifically, high intensity interval training (HIIT) has been shown to increase the efficiency of mitochondria in ATP production, as well as the number of mitochondria (Robinson et al), and it helps with mitophagy (Schiavi et al).
If we subject the system to stress and ask for energy from it to undertake muscle exercise this will lead to improved ATP synthesis, the creation of new mitochondria (biogenesis), the activation of autophagy (which allows for the recycling of damaged mitochondria, mitophagy), and it will increase ROS. Yes, I know that earlier in this article we spoke about the negative effect of ROS; however, here we have to introduce, just for a moment, the concept of mitohormesis: a small amount of damage because of the production of ROS, leading to improved mitochondrial function, signaling, and biogenesis (Son et al, Sena et al, Hekimi et al). When this production is excessive, damage to proteins and DNA occurs.
4. Hormones
The presence of estrogen and androgen receptors and hormone response elements in mitochondria led to research into the effects of 17β-estradiol and testosterone on mitochondrial functions and their relationship with aging. Both steroids trigger a complex molecular mechanism involving a cross-dialogue between the mitochondria, nucleus, plasma membrane, and cytoskeleton — leading to mitochondrial protection (Vasconsuelo et al).
BIBLIOGRAPHY
(1) Schiavi A, Ventura N. The interplay between mitochondria and autophagy and its role in the aging process. Exp Gerontol. 2014;56:147-153.
(2) D’Aquila P, Bellizzi D, Passarino G. Mitochondria in health, aging and diseases: the epigenetic perspective. Biogerontology. 2015;16(5):569-585.
(3) Robinson MM, Dasari S, Konopka AR, et al. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017;25(3):581-592.
(4) Son JM, Lee C. Mitochondria: multifaceted regulators of aging. BMB Rep. 2019;52(1):13-23. doi:10.5483/BMBRep.2019.52.1.300
(5) Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012; 48:158–167.
(6) Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011; 21:569–576
(7) Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012; 444:1–10
(8) Sahin E, Depinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012; 13:397–404.
(9) Gonzalez-Freire M, de Cabo R, Bernier M, et al. Reconsidering the Role of Mitochondria in Aging. J Gerontol A Biol Sci Med Sci. 2015;70(11):1334-1342.
(10) Vasconsuelo, A., Milanesi, L., & Boland, R. (2013). Actions of 17β-estradiol and testosterone in the mitochondria and their implications in aging. Ageing Research Reviews, 12(4), 907–917.
