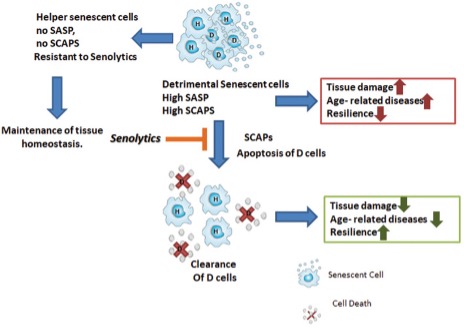
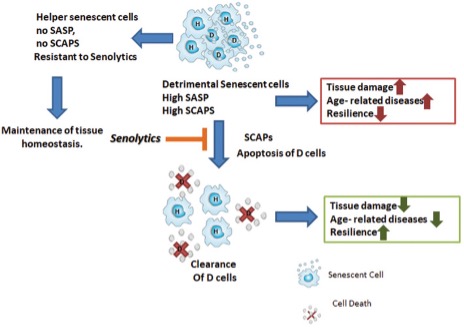
Cellular senescence is one of the 9 ‘‘Hallmarlks of Aging”. Some, but not all, senescent cells may acquire what we call a senescence-associated secretory phenotype (SASP).
Cellular senescence is related in one way or another to most diseases and syndromes associated with aging. We have the possibility of acting on it by influencing the aging process, the onset of diseases, and syndromes associated with age.
Dr. Alfonso Galán González – Neolife Medical Team
Introduction
Senescent cells are cells that do not die, but stop replicating, become “immortal” by resisting apoptosis (programmed cell death), and are often still very active. Hence the nickname “zombie cells”.
In this blog post, we’ll take an in depth look into one of those 9 “Hallmarks of aging”, determinants of aging, or molecular mechanisms of aging so often discussed in these texts: cellular senescence.
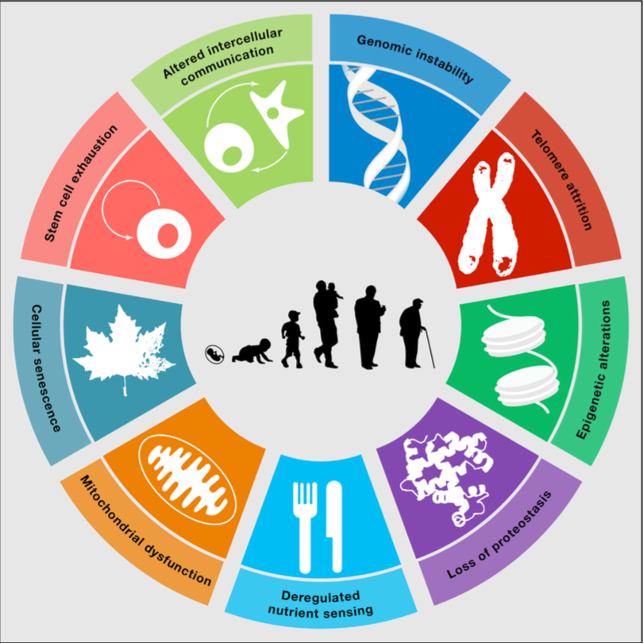
Why talk about cellular senescence?
- Cellular senescence is related in one way or another to the remaining 8 mechanisms.
- It is behind most diseases and syndromes associated with aging.
- We have the possibility of acting on it by influencing the aging process, the onset of diseases, and syndromes associated with age.
I believe that this more than justifies the relevance of this article and others to come, which will go into the strategies we have to combat it.
Aging
Aging is associated with the risk of developing multiple chronic diseases, geriatric syndromes, worsening physical resilience, and mortality. These risks increase exponentially in the last quarter of our lives.
Among the diseases for which age is a major risk factor we find:
Heart failure, myocardial infarction, dementia, stroke, cancer, diabetes and metabolic diseases, kidney dysfunction, chronic pulmonary disease, osteoporosis, arthritis, blindness, etc….
Geriatric syndromes include frailty, muscle loss, falls, incontinence, and moderate cognitive impairment, among others.
A worsened resilience manifests itself as slower recovery after heart attacks, strokes, injuries, surgeries, greater severity of infections like the flu or coronavirus, and a lower generation of antibodies after vaccination. The cause of all these pathologies and processes that limit human life and capabilities lies in these aging markers.
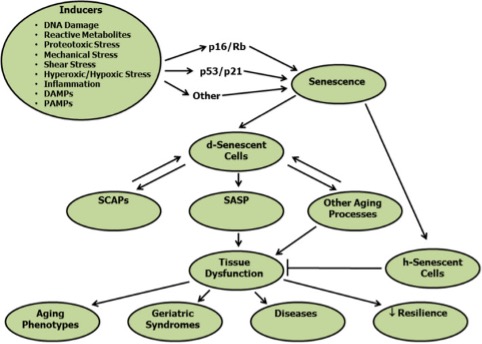
We know that cellular senescence contributes to the development of inflammation, fibrosis, DNA damage, development of protein aggregates, autophagy failure, lipotoxicity, mitochondrial dysfunction, NAD+ depletion, reactive oxygen species (ROS), and stem, progenitor, and immune cell dysfunction.
Cellular Senescence
Cellular senescence was first described in 1961 by Hayflick and Moorehead as a cellular fate of irreversible cessation of replication, maintained viability with resistance to apoptosis and, most often, an increase in metabolic activity. In other words, these are cells that do not die, but stop replicating, become “immortal” by resisting apoptosis (programmed cell death), and are often still very active.
These characteristics are what have led them to be colloquially called “zombie cells”. The causes that lead a cell to take this path are varied and include signals associated with tissue or cell damage and cancer development. These include:
- DNA damage
- Telomere shortening
- Exposure to extracellular DNA
- Activation of oncogenes
- Replication stress or proliferation inducers
- Protein aggregates and unstructured proteins
- Failure of protein recycling due to decreased autophagy
- Presence of AGEs (advanced glycation end products like hemoglobin)
- Saturated lipids or bioactive lipids like bradykinin or prostaglandins
- Reactive metabolites like ROS
- Mechanical stress like the one associated with the lack of cartilage in osteoarthritis or the one produced in atheroma plaques
- Inflammatory cytokines like TNF⍺
- DAMPS and PAMPS (damage associated molecular patterns and pathogen associated molecular patterns), i.e., intracellular contents released into the environment when bacterial cells or endotoxins are destroyed
Well, whatever the stimulus may be, one or more of the transcription factor cascades that promote this state of senescence (p16 INK4a, p53, p21, Rb, etc.) are activated.
From our cell culture data, it takes 10 days to 6 weeks for the cells to reach a complete state of senescence. This has important implications for treatment, as we will see when we discuss senolytics.
What makes the accumulation of senescent cells so bad, and why do we want to eliminate them? Some, but not all, senescent cells may acquire what we call a senescence-associated secretory phenotype (SASP). This means that these cells are going to secrete:
- Proinflammatory, proapoptotic, and insulin resistance-inducing cytokines like TNF⍺, IL6, IL8, and others
- Chemokines that attract, activate, and anchor immune cells
- Metalloproteases that cause tissue destruction
- Members of the TGFß family that lead to fibrosis and stem and progenitor cell dysfunction
- Activins and inhibins that also affect stem and progenitor cells
- Other factors like serpins that lead to thrombosis and fibrosis
- Growth factors that may lead to tumor dissemination
- Bioactive lipids like prostaglandins, bradykinin, and ceramides that lead to inflammation and tissue dysfunction
- MicroRNAs that also lead to stem and progenitor cell dysfunction, inflammation, and insulin resistance
- Exosomes that carry these cytotoxic and senescence-inducing signals to neighboring or distant sites
The senescent cells that acquire this secretory phenotype are 30 to 70% of those present, and the mediators they produce depend on the type of senescent cell and the cause that originated their conversion.
Cells that do not acquire this secretory phenotype are called “helper” cells that do not seem to release proinflammatory or proapoptotic factors, but the true utility of these cells and their interrelationship with the secretory ones has not yet been elucidated.
This secretory phenotype may be modulated by many factors such as hormonal environment, drugs, or pathogens.
Like everything in our bodies, it seems that the presence of senescent cells has some beneficial function; in fact, they have been associated with tissue remodeling during development and healing and even defense against cancer. Some research suggests that if we interfere with these senescence cascades, we may promote the development of cancer. However, getting rid of already formed senescent cells, many of which carry oncogenic mutations or lead to cancer formation because of their secretory phenotype, prevents or delays cancer development. Many studies using senolytic agents confirm this last point.
Although senescent cells may appear in human and other vertebrate tissues at any point in their lives, they accumulate in the later part of their lives. For example, cutaneous senescent cells increase dramatically from age 60 to 80 in otherwise healthy humans.
Senescent cells accumulate at the sites where many diseases originate throughout life. For example, in adipose tissue in diabetes and obesity, in the hippocampus and frontal cortex in Alzheimer’s, in the substantia nigra in Parkinson’s, in bones and bone marrow in osteoporosis, in lungs in pulmonary fibrosis, in the liver in cirrhosis, in the retina in AMD (age-related macular degeneration), in plaques in psoriasis, in kidneys in kidney disease, in the endothelium in preeclampsia, in the heart and arteries in cardiovascular diseases…. Interesting, isn’t it? This is certainly not a chance occurrence.
Precisely to demonstrate that this is not a coincidence, there are experiments in which senescent cells are injected to determine causality, i.e., to cause disease by introducing senescent cells into a tissue.
After introducing senescent cells into the knees of young mice, they caused a state of osteoarthritis with radiological and clinical findings similar to those of “natural” osteoarthritis. After injecting them with non-senescent cells, this did not happen. Other studies of the same type have caused frailty, a higher mortality, and kidney failure in healthy mice.
In our next article, we will discuss the drugs and supplements that are being investigated to selectively kill these senescent cells, which are called senolytics, and the fascinating results we have obtained so far.
BIBLIOGRAPHY
(1) Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518-536. doi:10.1111/joim.13141
(2) Robbins PD, Jurk D, Khosla S, et al. Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu Rev Pharmacol Toxicol. 2021;61:779-803. doi:10.1146/annurev-pharmtox-050120-105018
(3) Demaria M, Ohtani N, Youssef SA et al An essential role for senescent cells in optimal wound healing through secretion of PDGF‐AA. Dev Cell 2014; 31: 722–33
(4) Campisi J. Aging, cellular senescence, and cancer. Ann Rev Physiol 2013; 75: 685–705
(5) Xu M, Pirtskhalava T, Farr JN et al Senolytics improve physical function and increase lifespan in old age. Nat Med 2018; 24: 1246–56.10.1038/s41591-018-0092-9
(6) Chinta SJ, Woods G, Demaria M et al Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to parkinson’s disease. Cell Rep 2018; 22: 930–40.
(7) Sun S, Cai B, Li Y et al HMGB1 and Caveolin‐1 related to RPE cell senescence in age‐related macular degeneration. Aging 2019; 11: 4323–37.
(8) Xu M, Bradley EW, Weivoda MM et al Transplanted senescent cells induce an osteoarthritis‐like condition in mice. J Gerontol A Biol Sci Med Sci 2017; 72: 780–5.
(9) Kim SR, Jiang K, Ferguson CM et al Transplanted senescent renal scattered tubular‐like cells induce injury in the mouse kidney. Am J Physiol Renal Physiol 2020; 318: F1167–F76.